You are here
Home 🌿 Medical Cannabis News 🌿 Trial Finds THC/CBD Improved Quality Of Life In Glioma Patients 🌿Trial Finds THC/CBD Improved Quality Of Life In Glioma Patients
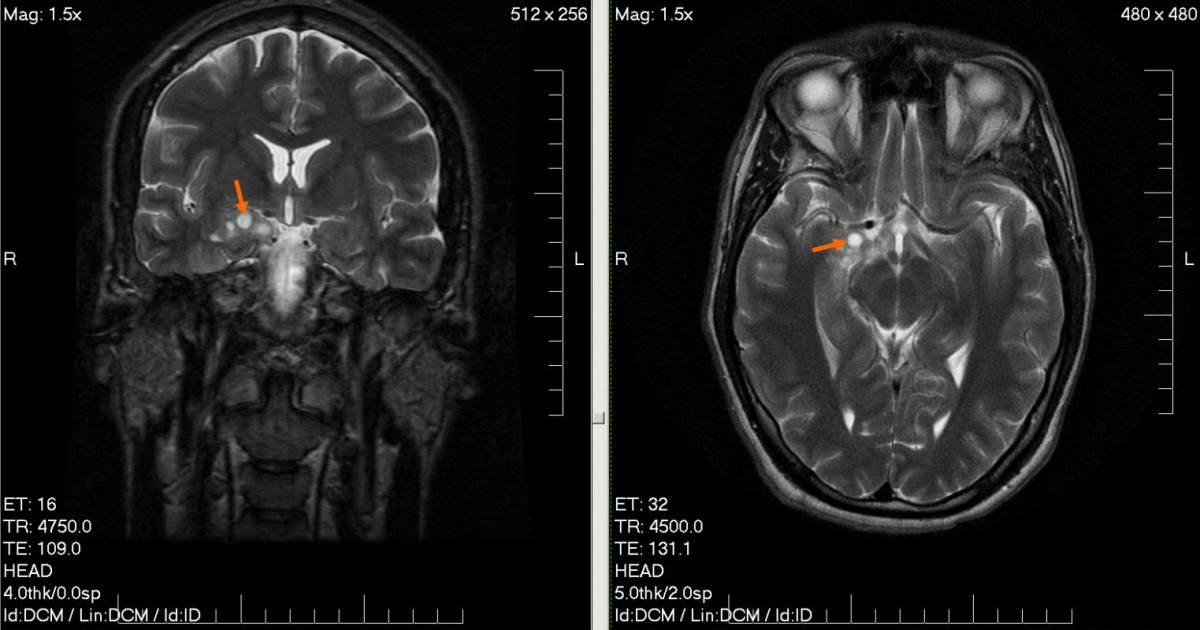
An Australian trial involving patients with glioma found medical cannabis preparations containing THC and CBD were well tolerated and provided a number of benefits.
Glioma is a type of tumor that can occur in the brain and spinal cord – it’s the most common primary brain tumour. There are several different types of glioma – astrocytomas, ependymomas and oligodendrogliomas.
A single-centre Phase II double-blind randomised clinical trial by researchers from the Prince of Wales Private Hospital, Australian Research Centre in Complementary and Integrative Medicine and other institutions investigated the tolerability of two different ratios of medicinal cannabis oil, taken orally, in patients who have been diagnosed with high grade gliomas* (GBM or astrocytoma AA3r) as an addition to their standard treatment.
We originally reported on this trial when it was announced back in late November 2018. It kicked off that month and continued through to December 2019.
The researchers concluded THC-containing medicinal cannabis was safe, involved no serious adverse effects and was well tolerated.
“Medicinal cannabis significantly improved sleep, functional wellbeing, and quality of life,” they state. “Our study data suggests that cannabis, especially a 1:1 CBD/THC mixture can be helpful for many of the symptoms impacting QoL in this patient population, especially sleep disturbance.”
Among the limitations of the trial acknowledged were no placebo group was used, which is considered gold standard in randomised-clinical trials
The trial results have been published in the journal Frontiers in Oncology and can be viewed here.
This study was supported financially by FIT-Bioceuticals Pty Ltd, Sydney, Australia and the products used were manufactured by Whistler Medical Marijuana Company, Canada.
* The image used for this article is of a low-grade brain glioma.
420 Intel is Your Source for Marijuana News
420 Intel Canada is your leading news source for the Canadian cannabis industry. Get the latest updates on Canadian cannabis stocks and developments on how Canada continues to be a major player in the worldwide recreational and medical cannabis industry.
420 Intel Canada is the Canadian Industry news outlet that will keep you updated on how these Canadian developments in recreational and medical marijuana will impact the country and the world. Our commitment is to bring you the most important cannabis news stories from across Canada every day of the week.
Marijuana industry news is a constant endeavor with new developments each day. For marijuana news across the True North, 420 Intel Canada promises to bring you quality, Canadian, cannabis industry news.
You can get 420 Intel news delivered directly to your inbox by signing up for our daily marijuana news, ensuring you’re always kept up to date on the ever-changing cannabis industry. To stay even better informed about marijuana legalization news follow us on Twitter, Facebook and LinkedIn.




